Search Results for " Daclatasvir "
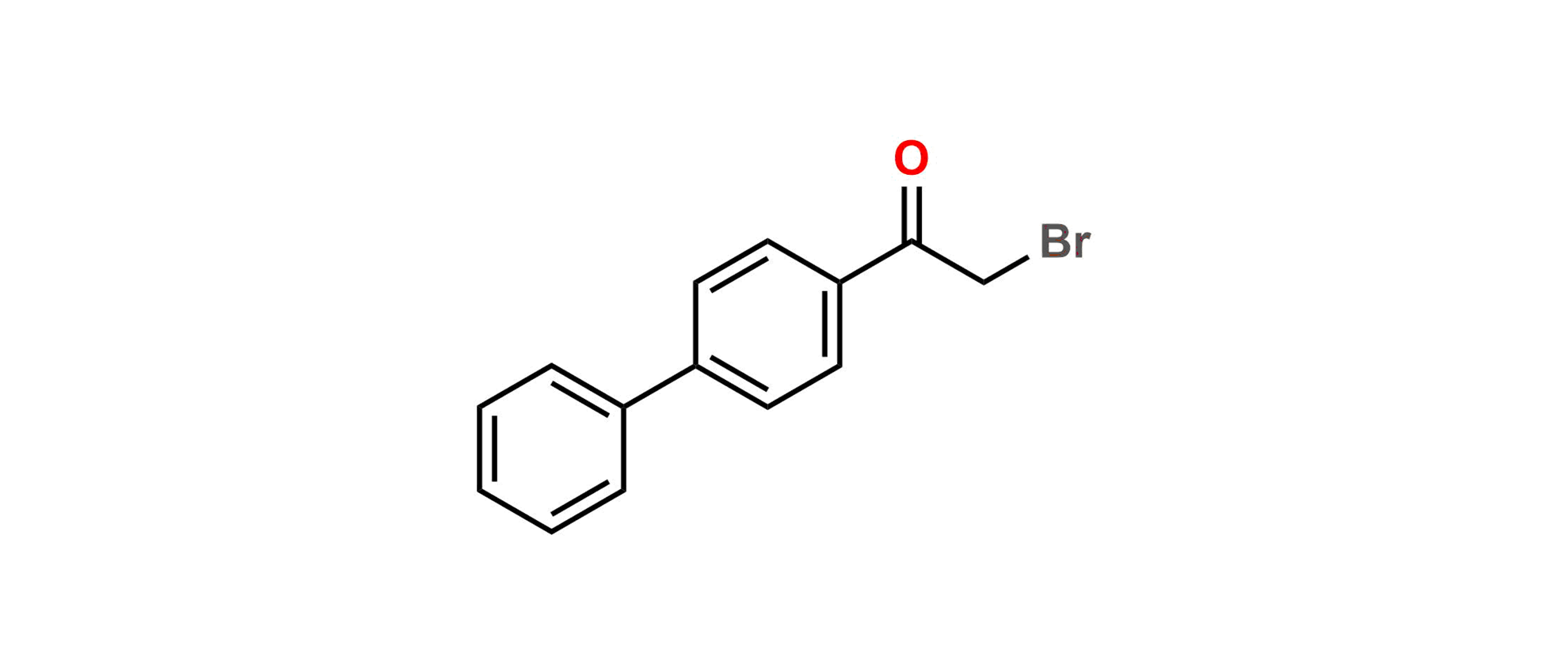
Daclatasvir Impurity 11
| SZ CAT No: | SZ-D036046 |
| CAS No | 135-73-9 |
| Mol.F. | C14H11BrO |
| Mol.Wt. | 275.1 |
| Inv. Status | Custom Synthesis |
Chemical Name: 1-([1,1'-Biphenyl]-4-yl)-2-bromoethan-1-one
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: O=C(C1=CC=C(C2=CC=CC=C2)C=C1)CBr
A validated stability-indicating reverse-phase high-performance liquid chromatography method for daclatasvir, identification and characterization of degradation products using LC-ESI-QTOF-MS
By Warghade, Snehal V.; Bothara, Kailash G.
From Asian Journal of Pharmaceutical and Clinical Research (2019), 12(5), 302-308
Development and validation of HPLC fluorescence and UPLC/DAD stability-indicating methods for determination of hepatitis C antiviral agent daclatasvir
By Kamal, Andra H.; Ismail, Nahla S.; Mabroijk, Mokhtar M.; Bebawy, Lories I.; Mekky, Mai A.
From Journal of AOAC International (2019), 102(4), 1125-1131
A stability-indicating UPLC method for the determination of potential impurities and its mass by a new QDa mass detector in daclatasvir drug used to treat hepatitis C infection
By Jagadabi, Varaprasad; Kumar, P. V. Nagendra; Mahesh, Kasthuri; Pamidi, Srinivasu; Ramaprasad, L. A.; Nagaraju, D.
From Journal of Chromatographic Science (2019), 57(1), 44-53
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase