Search Results for " Methotrexate "
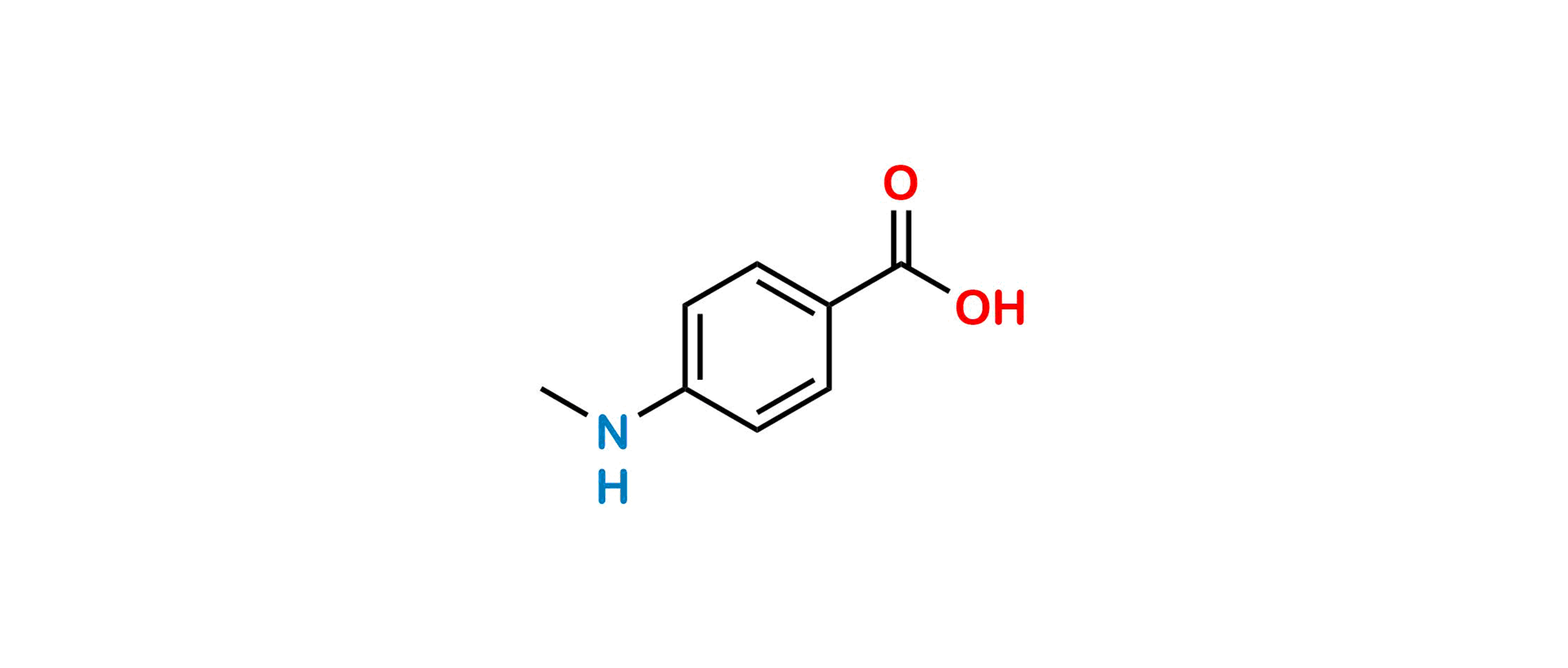
Methotrexate Impurity 9
| SZ CAT No: | SZ-M019042 |
| CAS No | 10541-83-0 |
| Mol.F. | C8H9NO2 |
| Mol.Wt. | 151.2 |
| Inv. Status | Custom Synthesis |
Chemical Name: 4-(Methylamino)benzoic acid
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: OC(C(C=C1)=CC=C1NC)=O
Identification and Quantitation of Impurities in Methotrexate
dulal c. chatterji, altice g. frazier, and joseph f. gallell
Journal of Pharmaceutical Sciences Volume 67, Issue 5, May 1978, Pages 622-624
determination of methotrexate and its major metabolite, 7_hydroxymethotrexate, using capillary zone electrophoresis and laser-induced fluorescence detection
mark c. roach, philippe gozel and richard n. zare
Journal of Chromatography, 426 (1988) 129-140
Analytical methodologies for determination of methotrexate and its metabolites in pharmaceutical, biological and environmental samples
Forough Karami , Sara Ranjbar , Younes Ghasemi , Manica Negahdaripour
Journal of Pharmaceutical Analysis 9 (2019) 373-391
Perspectives of Methotrexate-Based Radioagents for Application in Nuclear Medicine
Paweł Krzysztof Halik,* Przemysław Kozmin ́ ski, and Ewa Gniazdowska
Mol. Pharmaceutics 2021, 18, 1, 33–43
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase