Search Results for " Cefixime "
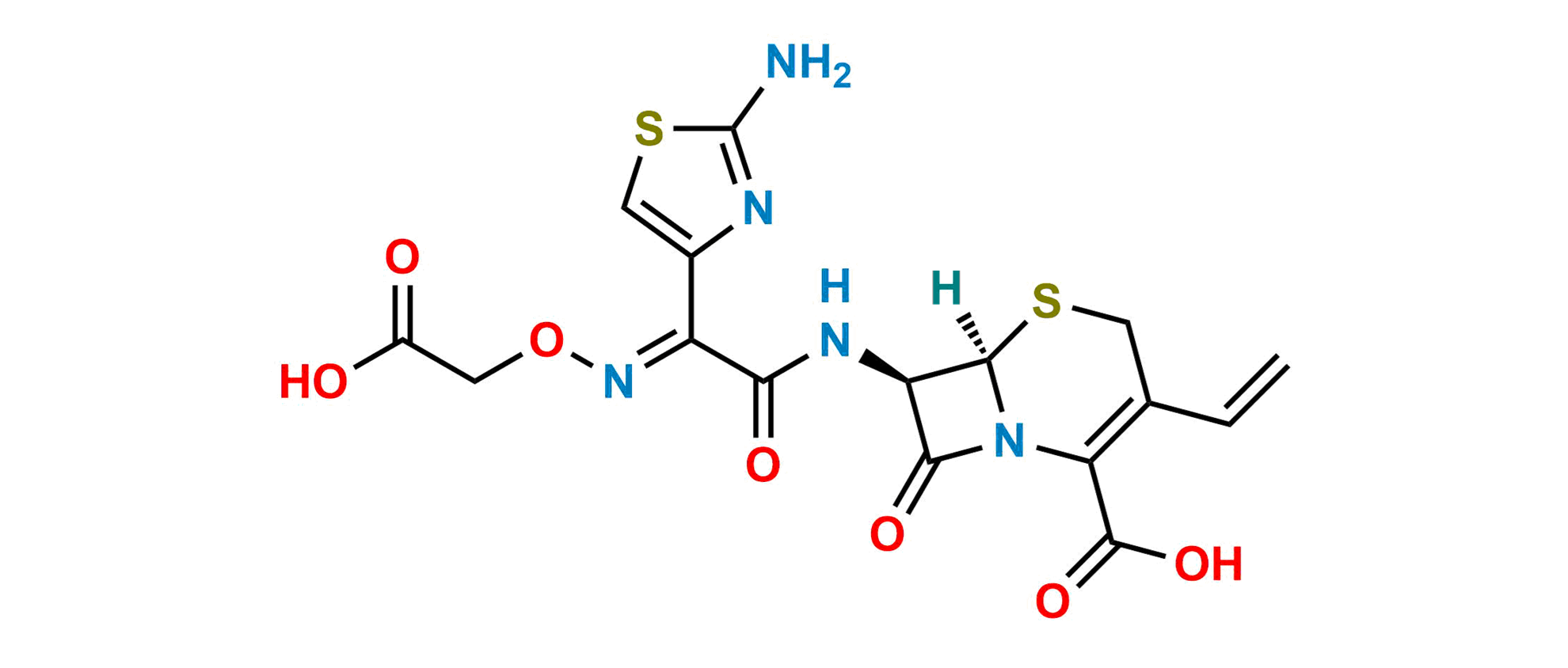
Cefixime EP Impurity D
| SZ CAT No: | SZ-C081005 |
| CAS No | 97164-56-2 |
| Mol.F. | C16H15N5O7S2 |
| Mol.Wt. | 453.4 |
| Inv. Status | In Stock, 3-4 days for Dispatch |
| Rel. CAS No | 79369-28-1 (HCl salt) ; 1202055-17-1 (Na salt) ; 1202055-18-2 (K salt) ; 210702-16-2 (Sulfate) |
| Shipping Condition | Ambient Temperature |
Chemical Name: (6R,7R)-7-[[(E)-2-(2-Aminothiazol-4-yl)-2-[(carboxymethoxy)imino]acetyl]amino]-3-ethenyl-8-oxo5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (as per EP)
Synonym: Cefixime (E)-Isomer (EP)
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: O=C(C(N12)=C(C=C)CS[C@]2([H])[C@H](NC(/C(C3=CSC(N)=N3)=N/OCC(O)=O)=O)C1=O)O
Analytical method development and validation of cefixime and azithromycin by using RP-HPLC
By Sireesha, E.; Reddy, L. Ramachandra; Dhachinamoorthi, D.
From World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(9), 1160-1177
Stability indicating hplc method for the quantification of cefixime, ornidazole and moxifloxacin in solid dosage forms
By Palacharla, Suresh Kumar; Mohan, G. V. Krishna
From Rasayan Journal of Chemistry (2018), 11(4), 1696-1714
Validated stability-indicating HPTLC method for cefixime and azithromycin with preparative isolation, identification, and characterization of degradation products
By Gawande, V. T.; Bothara, K. G.; Satija, C. O.
From Acta Chromatographica (2018), 30(4), 212-218
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase