Search Results for " Eslicarbazepine acetate "
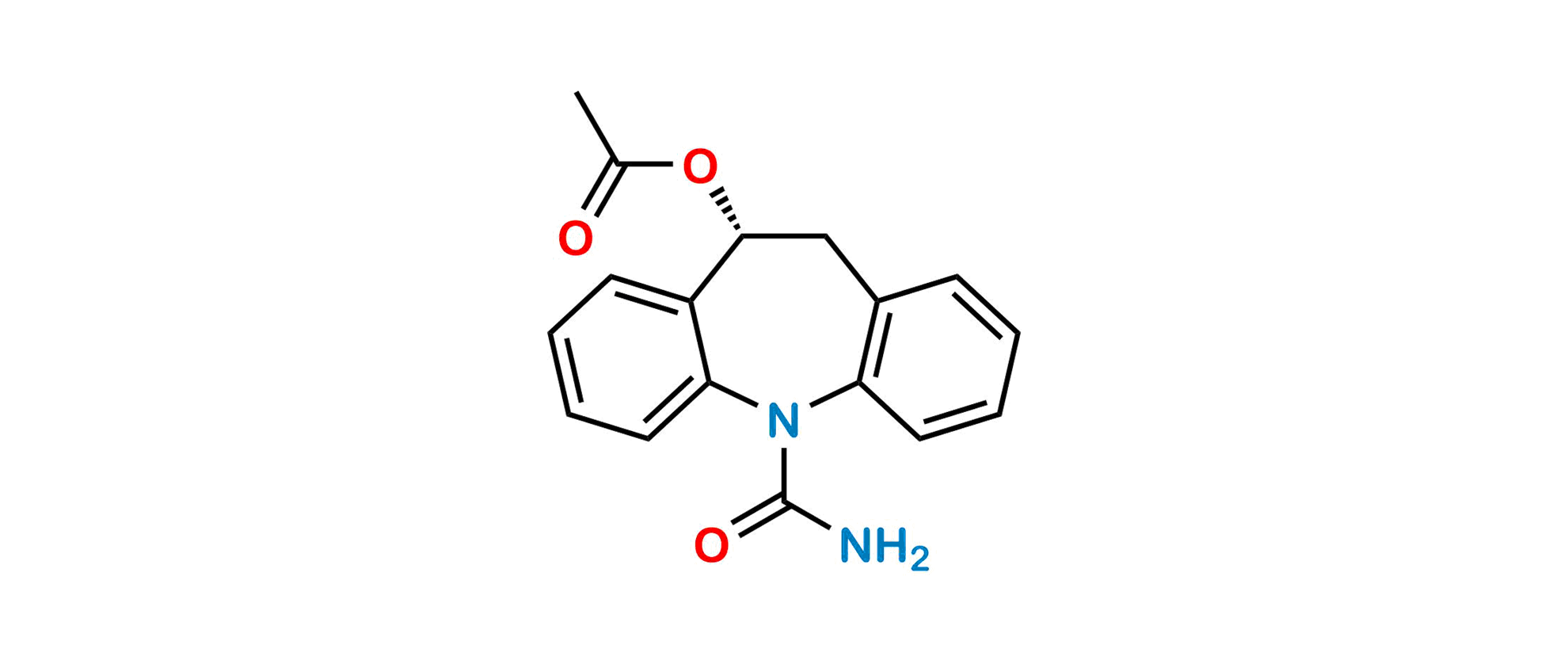
Eslicarbazepine R-Isomer
| SZ CAT No: | SZ-E034011 |
| CAS No | 186694-45-1 |
| Mol.F. | C17H16N2O3 |
| Mol.Wt. | 296.3 |
| Inv. Status | In Stock |
Chemical Name: (10R)-10-(Acetyloxy)-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide; (R)-(+)-10-Acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide; BIA 2-059
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: CC(O[C@H]1C2=CC=CC=C2N(C(N)=O)C3=C(C=CC=C3)C1)=O
Stability indicating hplc method for the determination of eslicarbazepine acetate and its impurities in bulk drugs and pharmaceutical dosage forms
Mudigonda Srinivas,1,2 Narasimha Rao Avupati,1 Shakil Sait,1 and K. Mukkanti
Journal of Liquid Chromatography & Related Technologies, 35:1550–1564, 2012
Enantioselective HPLC-UV method for determination of eslicarbazepine acetate (BIA 2-093) and its metabolites in human plasma
Gilberto Alves,1 Isabel Figueiredo,1 Margarida Castel-Branco,1 Ana Loureiro,2 Ana Fortuna,1 Amílcar Falcão1 * and Margarida Caramona
Biomedical chromatography Biomed. Chromatogr. 21: 1127–1134 (2007)
Stability indicating thin-layer chromatographic determination of eslicarbazepine acetate as bulk drug: Application to forced degradation study
Nikita V. Mali and Deepali A. Bansode
Der Pharmacia Lettre, 2016, 8 (11):38-47
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.


 Additional Documents required to purchase
Additional Documents required to purchase