Search Results for " Mebendazole "
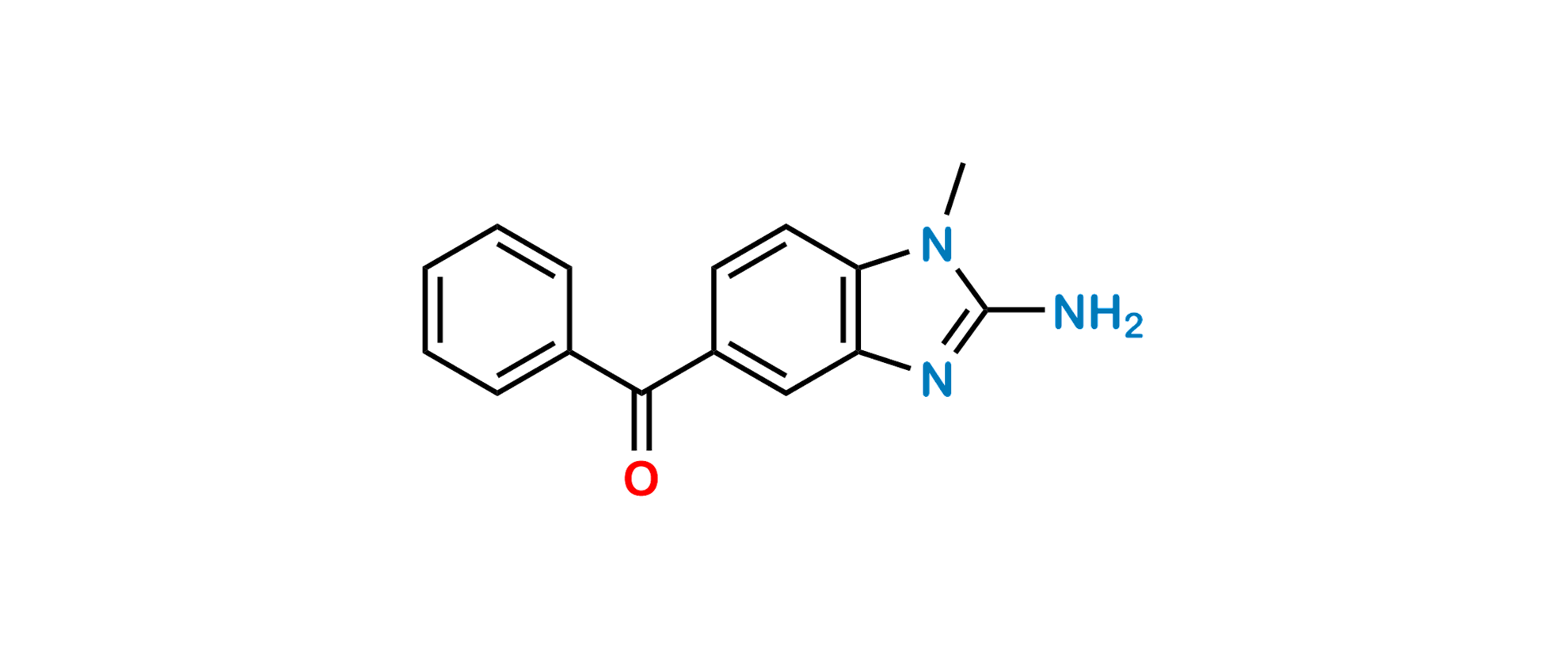
Mebendazole EP Impurity C
| SZ CAT No: | SZ-M010004 |
| CAS No | 66066-76-0 |
| Mol.F. | C15H13N3O |
| Mol.Wt. | 251.3 |
| Inv. Status | In Stock |
Chemical Name: 2-Amino-5-benzoyl-1-methylbenzimidazole; (2-Amino-1-methyl-1H-benzimidazol-5-yl)phenylmethanone; 1-Methyl-2-amino-5-benzoyl-(1H)benzimidazole;
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: O=C(C1=CC(N=C(N)N2C)=C2C=C1)C3=CC=CC=C3
High-performance liquid chromatographic separation and determination of the process related impurities of mebendazole, fenbendazole and albendazole in bulk drugs
Antony Raj Gomes *, V. Nagaraju
Journal of Pharmaceutical and Biomedical Analysis 26 (2001) 919–927
New RP-UPLC method development using QbD approach for determination of mebendazole, quinfamide, its impurities and antioxidants in mebendazole and quinfamide fixed dose combinations (FDC)
Rakesh Chandrakant Prabhu, Arthanareeswari Maruthapillai
Materials Today: Proceedings Volume 40, Supplement 1, 2021, Pages S120-S126
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase