Search Results for " Testosterone "
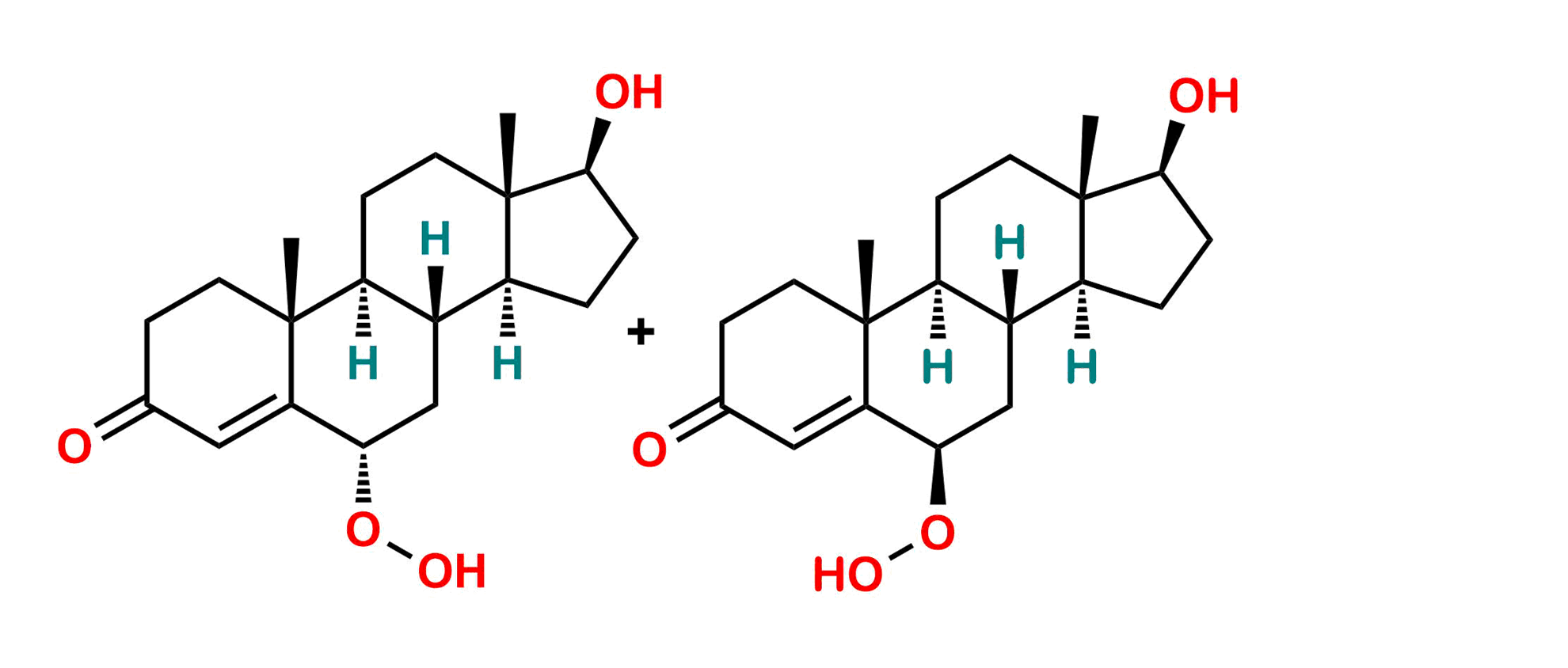
Mixure of 6α & 6β Hydroxyperoxy Testosterone
| SZ CAT No: | SZ-T021074 |
| CAS No | NA |
| Mol.F. | C19H28O5 |
| Mol.Wt. | 320.4 |
| Inv. Status | Custom Synthesis |
Chemical Name: (6R,8R,9S,10R,13S,14S,17S)-6-Hydroperoxy-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one compound with (6S,8R,9S,10R,13S,14S,17S)-6-hydroperoxy-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one (1:1)
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: O=C1CC[C@]2(C)[C@@]3([H])CC[C@]4(C)[C@@H](O)CC[C@@]4([H])[C@]3([H])C[C@H](OO)C2=C1.C[C@@](C([C@H](OO)C5)=CC6=O)(CC6)[C@]7([H])[C@]5([H])[C@@](CC[C@@H]8O)([H])[C@]8(C)CC7
Complete NMR assignment and absolute configuration of k4610422, a norditerpenoid inhibitor of testosterone-5α-reductase originally from Streptosporangium: rediscovery from a thermophilic Actinomadura
By Akiyama, Hirofumi; Oku, Naoya; Harunari, Enjuro; Panbangred, Watanalai; Igarashi, Yasuhiro
From Journal of Antibiotics (2020), 73(1), 60-65
Spectrophotometric and chromatographic strategies for exploring of the nanostructure pharmaceutical formulations which contains testosterone undecanoate
By Butnariu, Monica; Sarac, Ioan; Samfira, Ionel
From Scientific Reports (2020), Ahead of Print.
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase