Search Results for " Triamcinolone "
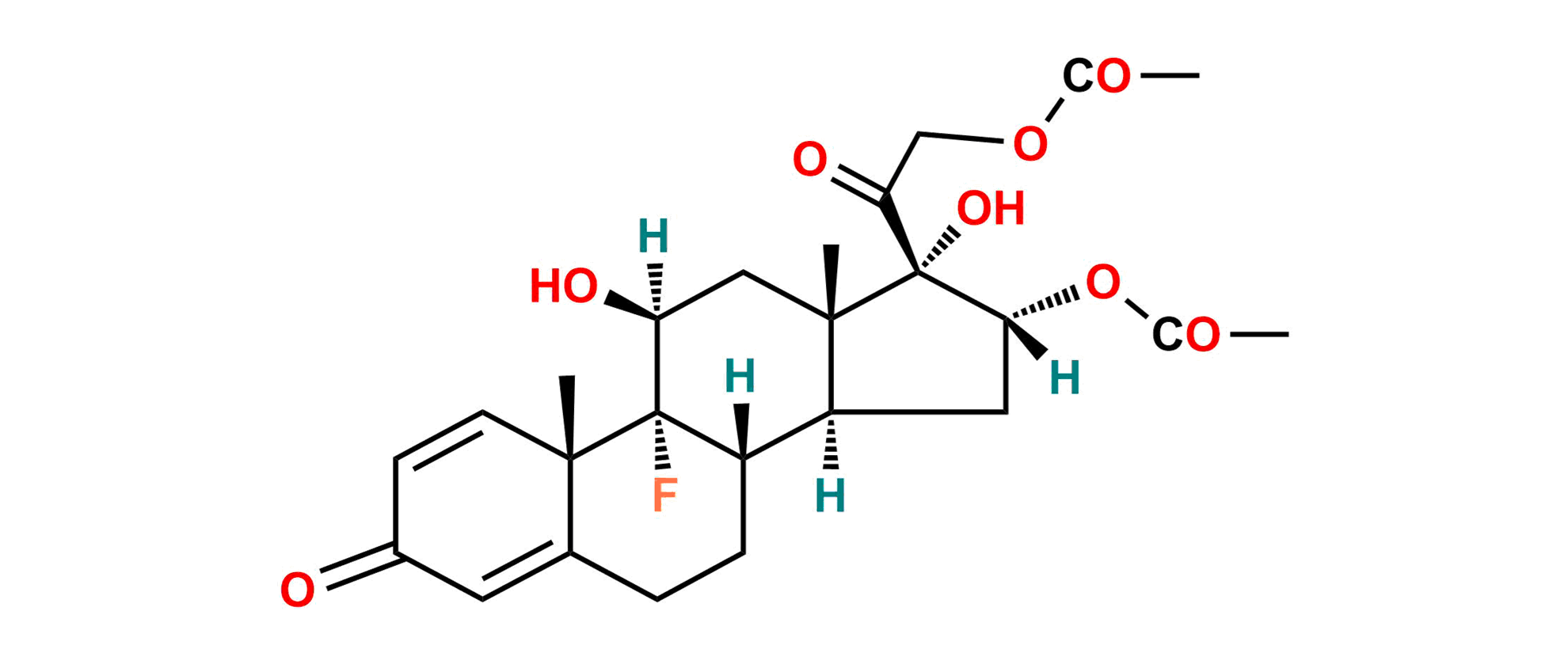
Triamcinolone EP Impurity A
| SZ CAT No: | SZ-T028002 |
| CAS No | 67-78-7 |
| Mol.F. | C25H31FO8 |
| Mol.Wt. | 478.5 |
| Inv. Status | In Stock |
Chemical Name: 9-Fluoro-11β,17-dihydroxy-3,20-dioxopregna-1,4-diene-16α,21-diyl diacetate (as per EP); 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 16,21-diacetate (as per USP)
Synonym: Triamcinolone Diacetate ; Triamcinolone 16,21-Diacetate
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: F[C@@]1([C@]2(C=C3)C)[C@](CCC2=CC3=O)([H])[C@@](C[C@](OC(C)=O)([H])[C@]4(O)C(COC(C)=O)=O)([H])[C@]4(C)C[C@]1([H])O
Δ14-steroidal impurity: preparation, characterization and evaluation, as well development of new HPLC method in drug substance like Triamcinolone Hexacetonide
By Shah, Tejas J.; Thakore, Anant R.; Chheda, Abhay H.; Desai, Geeta R.; Tandel, Harish S.
From Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry (2020), 8(1), 7-15
Resolution and quantitation of triamcinolone acetonide and its coformulated drug in the presence of its impurities and degradation products by HPTLC and HPLC
By Abbas, Samah S.; Hegazy, Maha A.; Hendawy, Hassan A. M.; Weshahy, Soheir A.; Abdelwahab, May H.
From Journal of AOAC International (2018), 101(4), 981-991
Development and validation of a stability-indicating HPLC-UV method for the determination of triamcinolone acetonide and its degradation products in an ointment formulation
By van Heugten, A. J. P.; de Boer, W.; de Vries, W. S.; Markesteijn, C. M. A.; Vromans, H.
From Journal of Pharmaceutical and Biomedical Analysis (2018), 149, 265-270
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

 Additional Documents required to purchase
Additional Documents required to purchase